
Orphan Drug Designation
December 30, 2022
Scientific Conference: “Quality Management By Cloud Computing: Comprehensive Solution Through MasterControl Application.”
February 27, 2023At present, technology transfer in general, and transfer analytical method, in particular, is a problems of great concern for factories or laboratories.

The transfer of analytical methods to improve product quality as well as bring domestic technology to ensure fast and sustainable development.
Before an analytical method is transferred, it should be properly developed and validated. The implementation of the transfer process, the analytical procedure, also known as the transfer of the analytical method, must be fully documented with the attached conditions. This article will discuss the key components required of the method transfer process between the receiving and transfer laboratories. Figure 1 describes analytical method transfer process.
Figure1: Analytical Method Transfer Professor
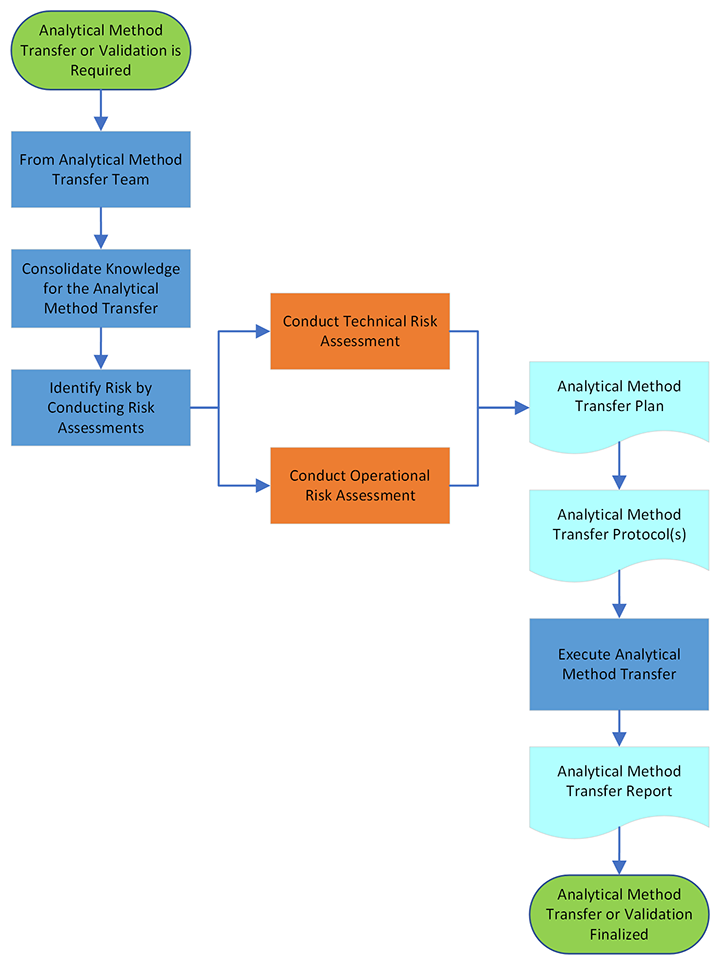
Each analytical method transfer between two laboratories involves two main parties: namely, the transferring laboratory (TL) and the receiving laboratory (RL). The transferring laboratory (TL) is the source or laboratory of origin for the analytical procedure, the receiving laboratory (RL) is the transfer laboratory’s recipient
Before transferring a test method, the transfer laboratory site should verify that the test method complies with those described in the marketing authorization or the relevant technical dossier.
The original validation of the test method(s) should be reviewed to ensure compliance with current requirements. Technical and operational risk assessments should be performed and documented to identify any supplementary validation that should be performed and from that to plan analytical method transfer.
An analytical method transfer plan that assesses time and resources, is recommended for the transfer of two or more methods. In some cases, when analytical method transfer is part of the transfer process, the analytical transfer method plan is included in the overall transfer plan.
When determining the transfer strategy, the following should be assessed: (1) Experience and knowledge of the receiving unit, (2) Degree of familiarity of the receiving unit with the methodology or technology used, (3) Specification of the product, and (4) Complexity of the analytical method. The analytical method transfer procedures may be different depending on the results of these assessments.
The nature of the method, the experimental set-up, and the data analysis must be tailored to the respective situation to ensure that regulatory requirements are met. A method transfer can be greatly simplified if, for example, the receiving laboratory (RL) already has experience with implementing a similar method, different strengths, or administration forms of the same product.
The following (Table 1) describes the different types of method transfer categories based on USP 1224:
Table 1: Different types of method transfer strategy with examples
| Type of strategy | Design | Examples |
| Comparative studies | The same sample is tested in both the TL and the RL and the results are then compared | LC/GC assay and related substances Other methods: e.g. water, residual solvents, ions, particle size distribution |
| Co-validation | From the outset, the RL is included in the validation of the method being transferred. | LC/GC assay and related substances |
| Revalidation | RL must performed revalidation or partial revalidation of the analytical method that is transferred | Microbiological testingOther critical threshold tests |
| Transfer waiver | Analytical procedures at RL are the same as TL | Can be considered for all method transfers but requires scientific justification since testing is not performed |
The analytical method transfer plan should include but is not limited to the following:
- Objectives
- The scope and responsibilities
- References
- Analytical Method Training
- Analytical Method Transfer Strategy
- Analytical Method Validation Strategy (as required)
- Qualification of Reference Standards
- Assessment of Critical Reagents
- Analytical Method Validation History
- Sample Testing Matrix
An analytical method transfer protocol is prepared detailed and approved between the transfer laboratory (TL) and the receiving laboratory (RL) which should include, but is not limited to, the following:
- Purpose
- Scope
- Materials and Equipment
- Experimental design
- References
- Appendices
The receiving laboratory performs the transfer according to the approved analytical method transfer protocol. Data review, and assessment of results should be performed by the transfer laboratory (TL) and the receiving laboratory with the following issues to be considered:
- Is the data being produced in the receiving laboratory considered fit for purpose?
- Is the analytical method operating reliably in the receiving laboratory?
- Are there any improvements to the analytical method to consider?
And in addition, deviations from the protocol should be investigated before the closure of the transfer process if applicable.
Acceptance criteria are also an important part of the successful transfer of analytical methods and must therefore be specified. The acceptance criteria should be based on the current validation study of the methodology and current requirements.
Thus, analytical method transfer is the most important part of a product transfer process, a decision on the success of the transfer process. The results of the transfer of analytical methods depend on many factors, so it requires careful preparation before the transfer to reach the goal of the successful transfer of analytical methods.
Documents references:
- USP <1224> Transfer of Analytical Procedures.
- ISPE Baseline Guide: Technology Transfer (Third Edition)




